The Lightning Bolt Symbol In The Cpt Manual Signifies What
Each year, a team of top disease experts and practice physicians work together to decide what changes will be made to the childhood immunization schedule, which helps protect U.S. Children and adults from diseases. The schedule (www.cdc.gov/vaccines/recs/schedules/child-schedule.htm#printable) is evaluated based on the most recent scientific data available. Changes are announced in January and approved by the American Academy of Pediatrics (AAP), the Centers for Disease Control and Prevention (CDC), and the American Academy of Family Physicians (AAFP). Providers receive notice of the changes, and are expected to purchase and administer the immunizations to fit the new schedule.Over the last several years new and expensive vaccines added to the schedule for adolescents, adults, and children have created a financial hardship for providers trying to balance economic pressures and best practices.
A survey commissioned by the National Vaccine Advisory Committee (NVAC) reported that 62 percent of decision makers in practices delayed purchase of a vaccine some time within the past three years due to financial concerns. The survey revealed that in the prior year 16 percent of practice decision makers seriously considered stopping vaccinations for privately-insured patients due to high cost and reimbursement issues. Offset High Costs with Appropriate BillingHow can practices continue to protect the communities they serve, prevent disease outbreak, and maintain financial viability? One way is to report the vaccines and vaccine-related services accurately, and bill appropriately. You should report vaccine administration using two families of CPT ® codes: One for the vaccine itself and one for the vaccine’s administration.To facilitate immunization reporting, the most recent new or revised vaccine product codes, resulting from recent CPT ® Editorial Panel actions, are published on the American Medical Association (AMA) CPT ® website on July 1 and Jan. 1 in a given CPT ® cycle. These dates correspond with CPT ® Editorial Panel meetings for each CPT ® cycle (June, October, and February).Watch for a lightning bolt symbol that was added to CPT ® in 2006 for vaccine codes pending approval from the Food and Drug Administration (FDA).
A full list is in Appendix K. These are normally not reimbursed until the FDA approves the vaccine but have been assigned codes pending approval, which often happens during that CPT ® cycle. Correct Vaccine ReportingUse CPT ® code range 8 to report the vaccine or toxoid product only, based on the produce manufacturer and brand, the specific schedule, chemical formulation, dosage, patient’s age, and/or route of administration. The exact vaccine provided must be reported this way to meet the requirements of immunization registries, vaccine distribution programs, and other reporting systems that track usage and administration of vaccines.With the dizzying array of vaccine producers and product names, it’s challenging to keep the CPT ® and ICD-9-CM codes straight. The AAP provides a free table with an easy-to-follow format allowing coders to access the correct CPT ® and ICD-9-CM code by knowing either the manufacturer or brand name. This resource can be found on the AAP website at Cautious with Combination DosesCodes are available for either individual vaccines or combination vaccines.
Combination vaccines are formulations of antigens that combine multiple vaccines into a single injection (for example, 90707 Measles, mumps and rubella virus vaccine (MMR), live, for subcutaneous use). It is not appropriate to report the components of a combination vaccine when a single CPT ® code exists to report the combination.Report Vaccine Administration SeparatelyCPT ® code range 4 reports the administration of the vaccine. Report these codes separately from the CPT ® code representing the vaccine product itself.
CPT SymbolsHere are the symbols commonly used in CPT reference coding books:. Bullet to the left of the code denotes this is a new code that's never been used before.Triangle - Located to the left of the code indicates the code description has been revised in the current edition of the CPT. Two Horizontal Triangles - These are around notes that have been revised.; - Semi-colon in a CPT description denotes that everything to the left of the semi-colon is applicable to the indented shorter descriptions following. Asterisk or star shows after minor surgery codes to show preoperative and postoperative services are included and means the package or global surgery is not applicable. These procedures are usually paid as fee-for-service.+ - Plus symbol identifies add-on codes usually performed at the same time and by the same provider as the primary procedure. There are usually notes in parenthesis to indicate the primary code(s) that these apply to.
Reference Material Symbol
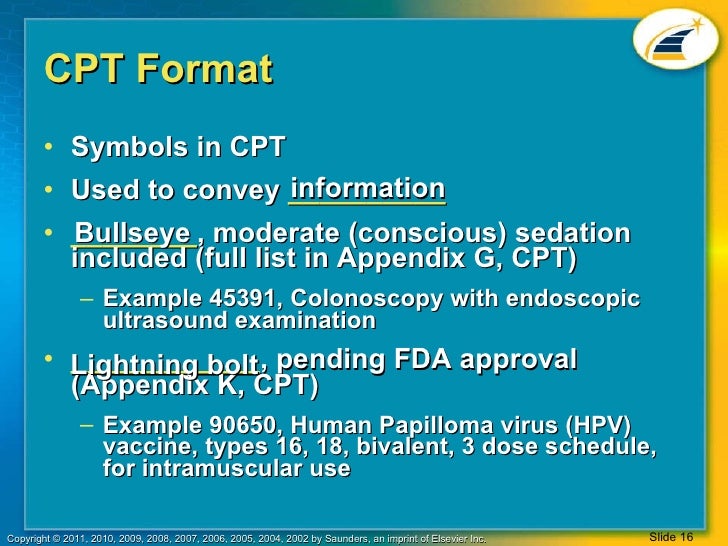
Circle with line through it - Means modifier -51 is not assigned to the code and are not add-on procedures. Lightning Bolt - Indicates product is pending FDA approval. Dot surrounded by Circle - Denotes moderate sedation.